Minerals
A mineral is a naturally occurring, inorganic substance of known chemical composition. Minerals are combined to make rocks.
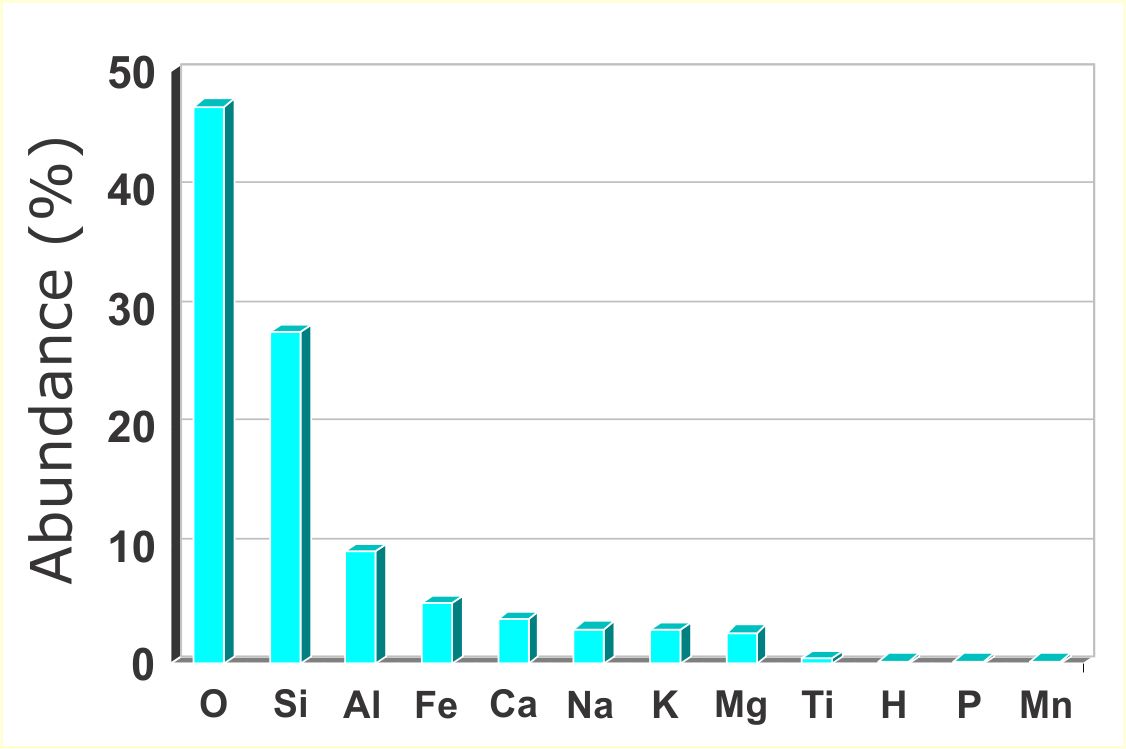
The most common chemical elements in the earth's crust are shown in the image on the right.
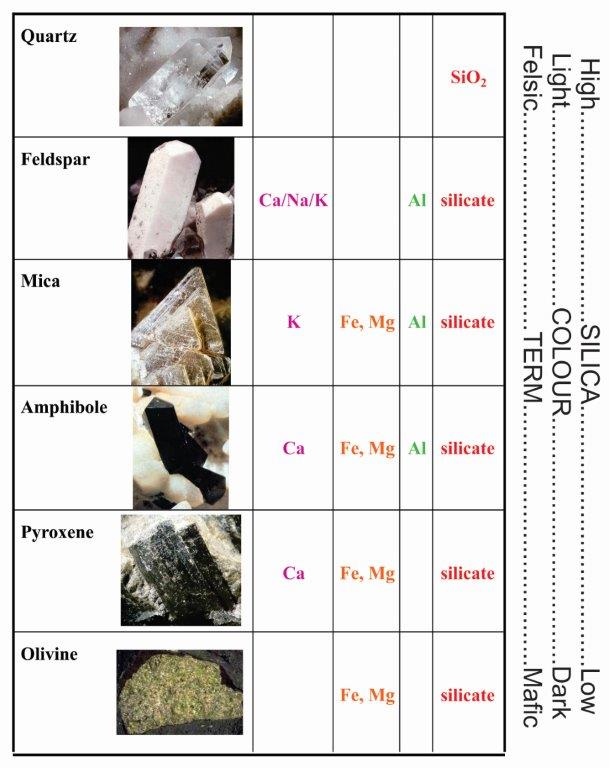
The two most common elements are Oxygen (O) and Silica (Si). If these elements are combined (two parts oxygen to one part silica) quartz (SiO2) is created. Minerals with quartz in them are known as silicates. Over 90% of the world’s minerals are silicates. A variety of common minerals can be created by adding a mixture of iron (Fe), magnesium (Mg), potassium (K), sodium (Na), calcium (Ca) and/or aluminium (Al) to quartz. For example, if we added iron (Fe) to quartz we would create a variety of the mineral called olivine. The most common minerals and their key ingredients are shown in the figures below.
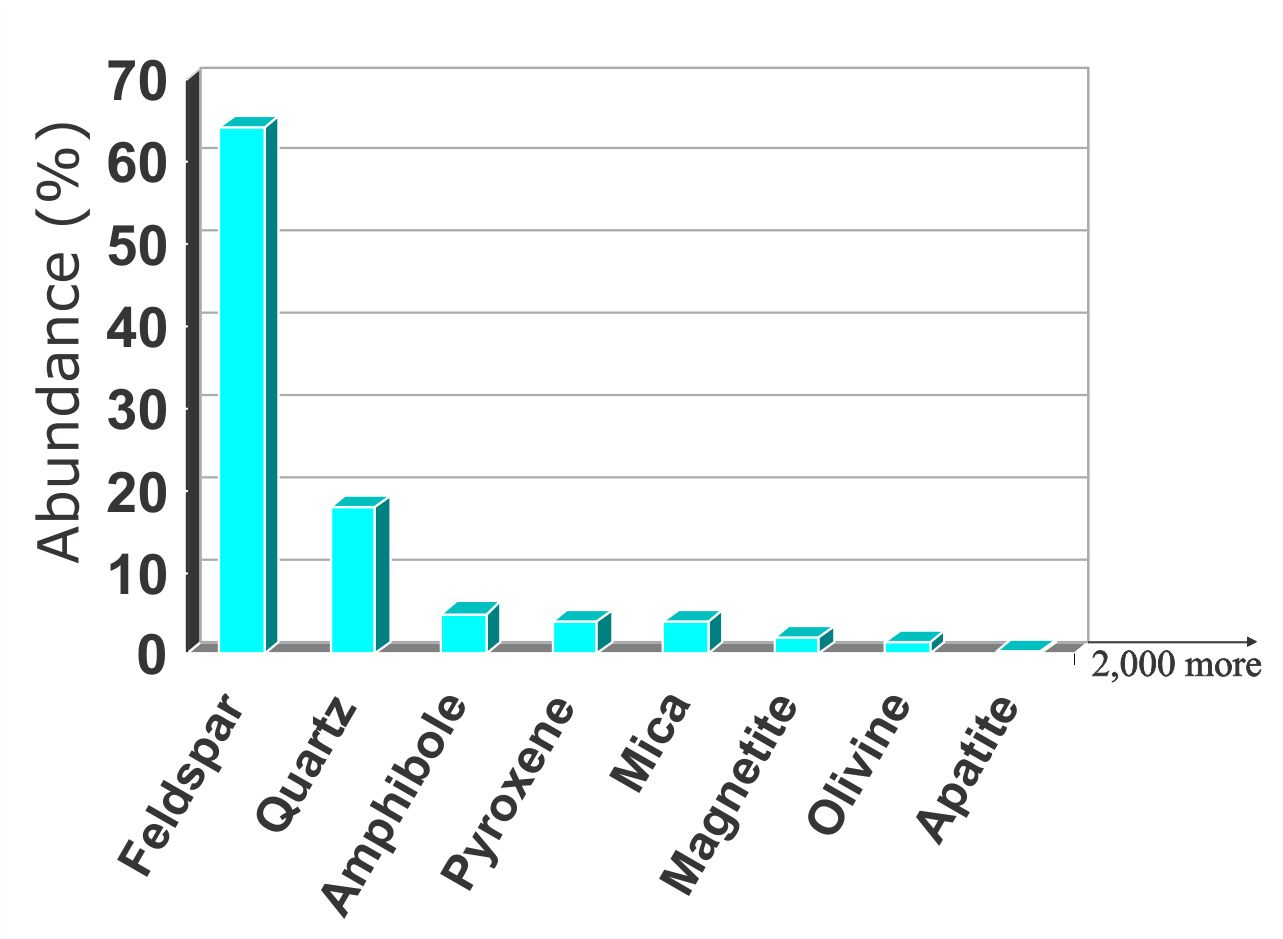
Minerals that are light in colour tend to have more Si, Ca, Na & K and these are referred to as felsic minerals. Minerals higher in Fe or Mg tend to be low in Si and are usually dark in colour. These are known as mafic minerals. Feldspar and quartz alone combine to make up almost all of the minerals in the earth's crust. Most rocks are made up of these common minerals. For example granites are mostly made up of feldspar, quartz, amphibole and mica. Yet despite the abundance of a few minerals, there are well over 5,000 known minerals.
Methods of classifying and identifying minerals
Geologists, like all scientists, have classification schemes in order to subdivide, classify and assist to understand the feature that they are studying. Many simple tests are very useful for identifying minerals. The differing characteristics listed below assist to classify minerals. Most classification schemes account for most of the minerals they are trying to cover. Beware though, geology is a science of exceptions. Sometimes interesting minerals don’t fit into simple classification schemes.
Colour – mafic (dark) or felsic (light)
As discussed previously and shown in the figure above, light coloured (felsic) minerals are most commonly high in Si, Ca, Na or K. However, there are numerous light coloured minerals that are not. Dark coloured (mafic) minerals are most commonly high in Fe or Mg, but there are numerous dark coloured minerals that are not. Yet colour is usually a good guide. It should be your first test. Is it mafic or is it felsic? If it is felsic it is most likely to be high in Si, Ca, Na or K, although be prepared that it may not be. If it is mafic it is most likely to be high in Fe or Mg, although be prepared that it may not be.
Colour - general
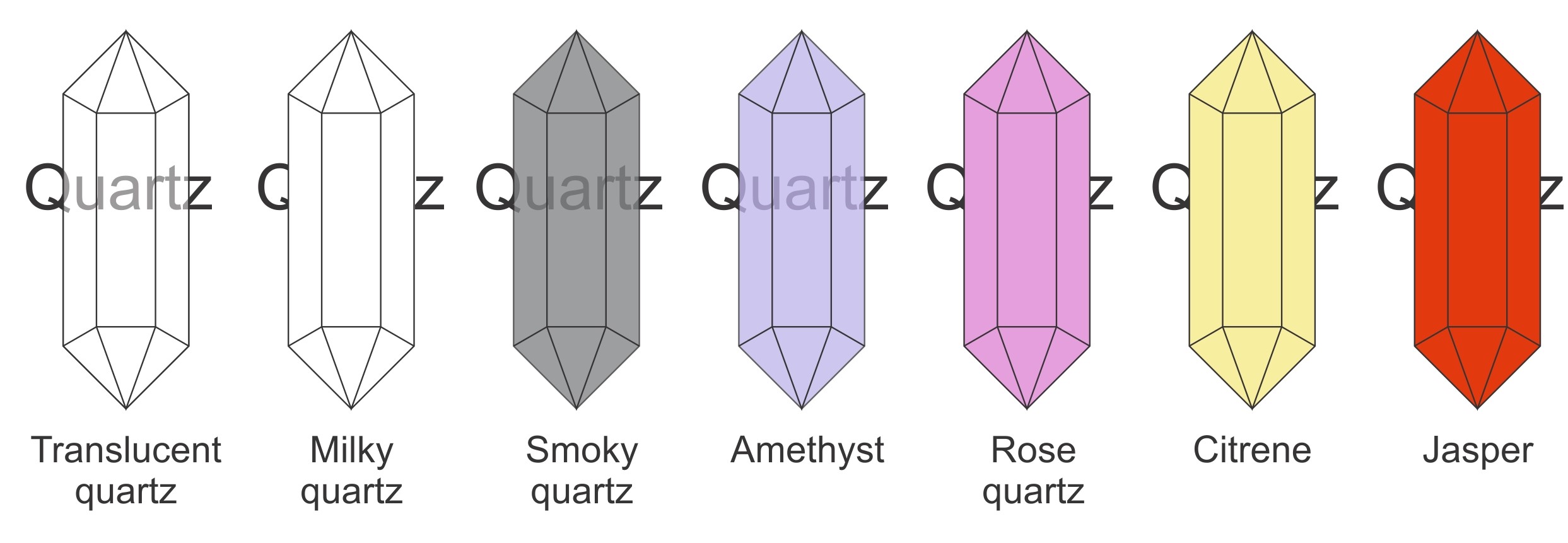
The overall colour of a mineral is the most obvious feature. Some colours are characteristic of certain minerals. However, it is common for the same mineral to be a variety of colours. For example, quartz can be variety of colours and can be either clear, white (milky), grey (smoky), purple (amythest), pink (rose), yellow (citrine), red-brown (jasper), white and pearly (calcedony), banded calcedony (agate). Corundum (Al2O3) is another mineral that comes in many colours. If transparent and red it is known as a ruby, if blue, green or yellow it is a sapphire. It can be very minor impurities that create the colour. For example, iron can make minerals red. Chromium makes rubies red. The impurities are often so minor that they do not change the overall chemistry of the mineral.
Streak
The true colour of a mineral is determined in powdered (crushed) form. The best way to achieve this is to scratch the mineral across the back of a tile (streak plate). Galena and haematite are both commonly grey in colour in hand specimen but have a grey and red streak respectively. Streak is one of the key tests that establishes a minerals identity. Further information - Wikipedia
Lustre
Lustre refers to the quality of the light reflecting from the crystal surface. Crystals can be transparent to semi-transparent, as shown above. However, the majority of minerals are non-transparent and can exhibit a metallic, sub-metallic or non-metallic lustre. The latter includes earthy (or dull), resinous, pearly, greasy, waxy or silky lustres. The term vitreous lustre is used for glassy minerals, like translucent quartz. The term adamantine (or brilliant) lustre is used for sparking reflections as found in diamonds. Further information - Wikipedia
Crystal systems
The differing manner in which atoms stack to create a mineral will create differing shapes. Regular packing of atoms may create a cubic shape. More elaborate packing may create hexagonal shapes as shown below. There are seven crystal systems, all minerals have one of these systems. Further information - Wikipedia


Cleavage and fracture
When a mineral breaks, it will either fracture erratically or split along planes of weakness within the crystal. Note the presence of crystal faces on the mineral does not necessarily mean it has cleavage. For example quartz will grow in nice hexagonal crystals but will fracture when hit with a hammer.
Cleavage can be distinctive for certain minerals. For example, mica’s will cleave in sheets, in the one direction. This is known as basal cleavage. Cleavage in two directions is known as prismatic, for example feldspar. Cleavage can form on three, four or six planes. Further information - Wikipedia
Crystal habit
Crystal habit is the external shape of individual crystals. Crystals habit can include cubic, bladed, columnar, tabular or acicular (needles). Further information - Wikipedia
Hardness
Some minerals are soft enough to be scratched by fingernails. A minerals hardness is it scratch resistance. A simple relative scale is established by the ability of harder minerals to scratch softer ones. Fingernails, copper coins, a knife, a metal file or glass can assist to establish hardness. Further information - Wikipedia
Density (specific gravity)
The density of a mineral relates to its weight compared to its size. Gold is very dense (19 g/cm3) whereas quartz is not (2.65 g/cm3). Further information - Wikipedia
Tenacity)
The tenacity of a mineral is it’s response to bending or breaking, they may be malleable, brittle, ductile, elastic or flexible. Further information - Wikipedia
Other properties of minerals
A variety of other properties may be distinguishable for certain minerals. Some may be magnetic or radioactive. Some may react to acids, fluoresce under ultraviolet light or conduct electricity. Minerals often burn differing colours under a flame. Some minerals, such as salt, have a certain taste. Others have a characteristic feel, such as greasy talc.